Chapter 2: Is Matter Around Us Pure? – Short Notes
1. Introduction
- A pure substance consists of a single type of particle (element or compound) and has a fixed composition and properties.
- A mixture contains more than one pure substance mixed together. Its composition is variable.
2. What is a Mixture?
- Types of Mixtures:
- Homogeneous Mixture: Has a uniform composition throughout. Its components are not visible and cannot be separated easily. (e.g., Salt solution, Sugar solution, Air).
- Heterogeneous Mixture: Does not have a uniform composition. Its components are visible and can be separated easily. (e.g., Mixture of salt and sand, Concrete, Ice cream).
3. Types of Pure Substances
- Element: A substance that cannot be broken down into simpler substances by chemical means. It is made of only one type of atom. (e.g., Gold (Au), Oxygen (O₂), Hydrogen (H₂)).
- Compound: A substance formed by the chemical combination of two or more elements in a fixed proportion. It can be broken down into its constituent elements only by chemical methods. (e.g., Water (H₂O), Carbon dioxide (CO₂), Sugar (C₁₂H₂₂O₁₁)).
- Difference between Mixtures and Compounds:
| Property | Mixture | Compound |
|---|---|---|
| Composition | Variable | Fixed |
| Properties | Properties of components are retained | New properties different from components |
| Separation | Components can be separated by physical methods | Components can be separated only by chemical methods |
| Formation | No energy change usually occurs | Energy (heat, light, etc.) is usually absorbed or released |
4. Solution, Suspension, and Colloid
A mixture can be classified based on the size of its particles.
| Property | Solution | Suspension | Colloid |
|---|---|---|---|
| Particle Size | < 1 nm | > 100 nm | 1 nm – 100 nm |
| Visibility | Not visible, cannot be scattered light | Visible, can be scattered light | Not visible, scatters light (Tyndall effect) |
| Homogeneity | Homogeneous | Heterogeneous | Heterogeneous but appears homogeneous |
| Stability | Stable | Unstable (settles down) | Stable |
| Separation | Cannot be separated by filtration | Can be separated by filtration | Cannot be separated by filtration |
| Example | Salt in water | Sand in water | Milk, Fog, Blood |
- Key Terms:
- Solute: The substance that is dissolved (e.g., salt in salt water).
- Solvent: The substance in which the solute is dissolved (e.g., water in salt water).
- Concentration of a Solution: The amount of solute present in a given amount of solution.
- Mass by mass percentage:
(Mass of solute / Mass of solution) × 100 - Mass by volume percentage:
(Mass of solute / Volume of solution) × 100
- Mass by mass percentage:
- Saturated Solution: A solution in which no more solute can be dissolved at a given temperature.
- Tyndall Effect: The scattering of a beam of light by colloidal particles, making the path of light visible. This is why we see a beam of sunlight in a dusty room or through fog.
5. Physical and Chemical Changes
- Physical Change: A change in which no new substance is formed. It is usually reversible. (e.g., Melting of ice, Dissolving sugar in water).
- Chemical Change: A change in which one or more new substances with new properties are formed. It is usually irreversible. (e.g., Burning of paper, Rusting of iron).
6. Separation Techniques
Different methods are used to separate components of a mixture based on their properties.
| Technique | Principle | Example of Use |
|---|---|---|
| Evaporation | To separate volatile solvent from non-volatile solute | Obtaining salt from sea water |
| Centrifugation | Separating denser particles from lighter ones by spinning | Separating butter from cream |
| Separation Funnel | Difference in densities of two immiscible liquids | Separating oil and water |
| Sublimation | Direct conversion of solid to vapour without melting | Separating ammonium chloride from sand |
| Chromatography | Different solubilities and adsorption of components | Separating dyes in ink |
| Distillation | Difference in boiling points | Purifying water, Separating alcohol and water |
| Fractional Distillation | Difference in boiling points of miscible liquids | Separating components of petroleum, air |
| Crystallisation | Obtaining pure crystals from an impure sample | Purification of salt or copper sulphate |
- Fractional Distillation: Used when the difference in boiling points is less than 25 K. A fractionating column is used to provide many surfaces for vaporisation and condensation.
Important Questions & Answers
Very Short Answer Type Questions (1 Mark)
1. Define a solution.
Ans: A solution is a homogeneous mixture of two or more substances. The component present in a smaller amount is called the solute, and the one present in a larger amount is the solvent.
2. What is the Tyndall effect?
Ans: The scattering of a beam of light by colloidal particles present in a colloid, making the path of light visible, is called the Tyndall effect.
3. Give an example of a gaseous solution.
Ans: Air (a homogeneous mixture of gases like nitrogen, oxygen, CO₂, etc.).
4. Name the process used to separate butter from cream.
Ans: Centrifugation.
5. What is the concentration of a solution?
Ans: The amount of solute dissolved in a given quantity of the solution is called the concentration of the solution.
6. What is a saturated solution?
Ans: A solution in which no more solute can be dissolved at a given temperature is called a saturated solution.
7. Is air a mixture or a compound?
Ans: Air is a homogeneous mixture because its composition is not fixed and its components can be separated by physical means.
Short Answer Type Questions (2-3 Marks)
1. Differentiate between homogeneous and heterogeneous mixtures with examples.
Ans:
| Homogeneous Mixture | Heterogeneous Mixture |
|---|---|
| Has a uniform composition throughout. | Does not have a uniform composition. |
| Components are not visible to the naked eye. | Components are visible. |
| No distinct boundaries between components. | Has distinct boundaries. |
| Example: Salt solution, Air. | Example: Mixture of salt and sand, Concrete. |
2. List the points of differences between a compound and a mixture.
Ans: (Refer to the table in the short notes section). Mention composition, properties, separation, and energy changes.
3. How will you separate a mixture of common salt and ammonium chloride?
Ans: We can separate them using the process of sublimation. Ammonium chloride is a sublime substance, whereas common salt is not. On heating the mixture, ammonium chloride will directly convert into vapour, which can be cooled and collected separately. Common salt will be left behind.
4. Why is water called a universal solvent?
Ans: Water is called a universal solvent because it can dissolve a large number of substances (salts, sugars, gases, etc.) in it to form solutions. This is due to its high polarity.
5. Calculate the concentration of a solution which contains 2g of salt dissolved in 50g of water.
Ans:
- Mass of solute (salt) = 2g
- Mass of solvent (water) = 50g
- Mass of solution = Mass of solute + Mass of solvent = 2g + 50g = 52g
- Concentration (mass by mass %) = (Mass of solute / Mass of solution) × 100
= (2 / 52) × 100 ≈ 3.84%
Long Answer Type Questions (5 Marks)
1. a) What is chromatography? Explain its principle.
b) How will you separate the components of black ink using chromatography?
Ans:
a) Chromatography is a technique used to separate those solutes that dissolve in the same solvent. Its principle is based on the differential adsorption of different components of a mixture on an adsorbent (like filter paper). The components move at different speeds on the paper, causing them to separate.
b) Activity to separate ink:
- Take a strip of filter paper and draw a line with black ink near the bottom.
- Pour a small amount of water (solvent) into a beaker, ensuring the water level is below the ink line.
- Hang the paper strip in the beaker so that the ink dip is just above the water level.
- As water rises up the paper, it carries the components of the ink with it.
- Different dyes in the ink will dissolve differently in water and will be adsorbed differently on the paper, leading to the separation of various coloured bands.
2. With a labelled diagram, describe the method of fractional distillation. Write its two applications.
Ans:
- Method: Fractional distillation is used to separate a mixture of two or more miscible liquids with boiling point differences less than 25 K.
- Process: The mixture is heated in a distillation flask. The vapour of the more volatile liquid (lower boiling point) rises first. In the fractionating column, the vapours condense and re-vaporise multiple times. With each vaporisation, the vapour becomes richer in the more volatile component. Finally, the pure vapour of the more volatile liquid passes into the condenser, where it is liquefied and collected. The less volatile component remains in the flask.
- Applications:
- Separation of different fractions (like petrol, diesel, kerosene) from crude petroleum.
- Separation of different gases from liquid air (like oxygen, nitrogen, argon).
3. Differentiate between physical and chemical changes. Give three examples of each.
Ans:
- Physical Change: A change in which no new substance is formed and the change is usually reversible.
- Examples: Melting of ice, Tearing of paper, Dissolving sugar in water.
- Chemical Change: A change in which one or more new substances with entirely new properties are formed. The change is usually irreversible.
- Examples: Burning of wood, Rusting of iron, Cooking of food.
Is Matter Around Us Pure InText Questions and Answers
Page – 15
Question 1.
What is meant by a pure substance?
Answer:
A pure substance is the one that consists of a single type of particle, i.e., all constituent particles of the substance have the same chemical nature. Pure substances can be classified as elements or compounds.
Board Exam Guides
Question 2.
List the points of difference between homogeneous and heterogeneous mixtures.
Answer:
Homogeneous mixtures:
- Homogeneous mixtures have uniform composition.
- It has no visible boundaries of separation between its constituents.
Heterogeneous mixtures:
- Heterogeneous mixtures have non-uniform composition.
- It has visible boundaries of separation between its constituents.
Page – 18
Question 3.
Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer:
A homogeneous mixture is a mixture having a uniform composition throughout the mixture. For example, mixtures of salt in water, sugar in water, copper sulphate in water, iodine in alcohol, alloy and air have uniform composition throughout the mixtures.
On the other hand, a heterogeneous mixture is a mixture having a non-uniform composition throughout the mixture. For example, composition of mixtures of sodium chloride and iron filings, salt and sulphur, oil and water, chalk powder in water, wheat flour in water, milk and water are not uniform throughout the mixtures.
Question 4.
How are sol, solution and suspension different from each other?
Answer:
Types of Mixtures: Sol, Solution, and Suspension
Sol
- Sol is a heterogeneous mixture.
- The solute particles are very small, not visible to the naked eye, and appear uniformly spread throughout the mixture.
- Tyndall effect is observed in this mixture.
Example: Milk of magnesia, mud.
Solution
- Solution is a homogeneous mixture.
- The solute particles dissolve completely and spread uniformly throughout the mixture.
- Tyndall effect is not observed in this mixture.
Example: Salt in water, sugar in water, iodine in alcohol, alloy.
Suspension
- Suspension is a heterogeneous mixture.
- The solute particles are visible to the naked eye and remain suspended throughout the medium.
- Tyndall effect is observed in this mixture.
Example: Wheat flour in water, chalk powder in water.
Question 3.
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:
Concentration means amount of a solute dissolved in 100 g of the solvent. Here, 36 g of sodium chloride is dissolved in 100 g of water, therefore, concentration of the solution is 36 g per 100 g of water.
Page – 24
Question 4.
How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other?
Answer:
Kerosene and petrol and miscible liquids also the difference between their boiling point is more than 25°C so they can be separated by the method of distillation.
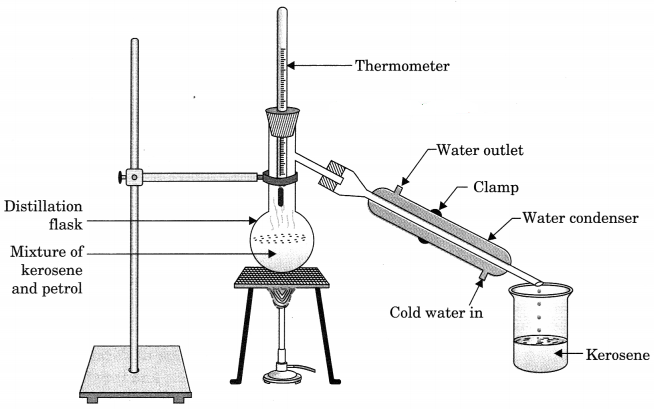
In this method, the mixture of kerosene and petrol is taken in a distillation flask with a thermometer fitted in it. We also need a beaker, a water condenser, and a Bunsen burner. The apparatus is arranged ‘ as shown in the above figure. Then, the mixture is heated slowly. The thermometer should be watched simultaneously. Kerosene will vapourise and condense in the water condenser. The condensed kerosene is collected from the condenser outlet, whereas petrol is left behind in the distillation flask.
Question 5.
Name the technique to separate
(i) butter from curd
(ii) salt from sea water
(iii) camphor from salt.
Answer:
(i) Butter can be separated from curd by centrifugation.
(ii) Salt can be separated from sea water by evaporation.
(iii) Camphor can be separated from salt by sublimation.
Question 6.
What type of mixtures are separated by the technique of crystallisation?
Answer:
Solids, in which the impurities are either insoluble or are more soluble than the solids in a particular solvent, can be easily separated by the technique of crystallisation. For example, impure copper sulphate, alum, nitre, sea salt, etc., can be easily purified by the technique of crystallisation.
Page – 24
Question 7.
Classify the following as chemical or physical changes:
(i) cutting of trees,
(ii) melting of butter in a pan,
(iii) rusting of almirah,
(iv) boiling of water to form steam,
(v) passing of electric current through water and the water breaking down into hydrogen and oxygen gases,
(vi) dissolving common salt in water,
(vii) making a fruit salad with raw fruits, and
(viii) burning of paper and wood.
Answer:
(i) Physical change
(ii) Physical change
(iii) Chemical change
(iv) Physical change
(v) Chemical change
(vi) Physical change
(vii) Physical change
(viii) Chemical change.
Question 8.
Try segregating the things around you as pure substances or mixtures.
(a) distilled water
(b) curd
(c) diamond
(d) ice-cream
(e) kerosene oil
(f) cooking oil
(g) steel
(h) graphite
(i) raw rubber
(j) vulcanized rubber
(k) solder wire.
Answer:
Pure substances – Distilled water, diamond, graphite, raw rubber.
Mixtures – Curd, ice-cream, kerosene oil, cooking oil, steel, vulcanized rubber, solder wire (alloy of lead and tin).
In-Text Activities Solved
( Textbook Page 1)
Activity 2.1
Answer:
Observation: Group A and B have obtained a mixture which has a uniform composition throughout. Such mixtures are called homogeneous mixtures or solutions. Though both the groups, i.e., A and B have obtained copper sulphate solution but the intensity of colour of the solutions is different. Different intensity of colour of the solutions shows that a homogeneous mixture can have a variable composition. Groups C and D have obtained mixtures, which contain physically distinct parts and have non-uniform compositions. Such mixtures are called heterogeneous mixtures.
Conclusion: A mixture which has a uniform composition throughout. Such mixtures are called homogeneous mixtures or solutions.
A mixture which has variable composition throughout. Such mixtures are called heterogeneous mixtures or solutions.
(Textbook Page 15)
Activity 2.2
Answer:
Observation:
- The particles in case of group A and B will not be visible whereas in group C and D it would be visible.
- In case of group A and B, the particles will not show path of the beam of light whereas in group C and D the particles will show the path of beam of light from a torch.
- The mixture is stable only in case of group A and B, as it does not settle down when kept for some time.
- For D it is not observed rapidly. On filtering the mixture, residue is left on the filter paper in case of group C.
Conclusion: Groups A and B have true solutions. Group C has suspension. Group D has got a colloidal solution.
(Textbook Page 16)
Activity 2.3
Answer:
Observation: When no more solute can be dissolved in a solution at a given temperature, it is called a saturated solution. So, at any particular temperature, a solution that has dissolved as much solute as it is capable of dissolving is said to be a saturated solution.
No, the amount of salt and sugar or barium chloride that can be dissolved in water at a given temperature is not the same. By increasing the temperature more solute can be added.
Conclusion: The amount of the solute present in the saturated solution at the given temperature is called its solubility. We can infer from the activity that different substances in a given solvent have different solubilities at particular temperature.
(Textbook Page 19)
Activity 2.4
Answer:
Observation: The liquid present in the watch glass evaporates on heating. A residue of ink will be left on the watch glass in a dry state. We can separate the volatile component (solvent) from its non-volatile solute by the method of evaporation. Ink is a mixture of a dye in water or a solvent.
Conclusion: From this activity we can conclude that ink is not a single substance, but is a mixture of dye and water/ solvent.
(Textbook Page 19)
Activity 2.5
Answer:
Observation: The number of soluble fats present in the milk collides with one smother at high speed to form bigger particles in the form of cream. The cream formed get separated and leaves behind the fat free milk. Centrifugation works on the principle that the denser particles are forced to move to the bottom and the lighter particles stays at the top of the test tube when spin rapidly.
Class 7 Solutions
Conclusion: Cream can be separated from milk by the process of centrifugation.
(Textbook Page 20)
Activity 2.6
Answer:
Observation: Two distinct layers of kerosene oil and water are formed when separating funnel is left undisturbed for some time. This process is based on the principle that immiscible liquids separate out in layers depending on their densities. Heavier density liquid remains in bottom as lower layer while the lighter density liquid present as upper layer.
Conclusion: A separating funnel helps in separating two immiscible liquids based on the difference in the densities of the liquids in the mixture.
(Textbook Page 21)
Activity 2.7
Answer:
Observation: The ink that we use has water as the solvent and the dye is soluble in it and so when the water rises on the filter paper it takes along with it the dye particles. Yes, different coloured spots of dyes are obtained because a dye is a mixture of two or more colours. The coloured component is more soluble in water; therefore it rises faster on the paper strip.
Conclusion: Coloured components of a mixture can be separated by using chromatography.
Class 7 Solutions
(Textbook Page 21)
Activity 2.8
Answer:
Observation: The thermometer shows an increase in the temperature. At 56.5°C, the thermometer reading becomes constant for some time. At this temperature, acetone vapourises, condenses and can be collected from the condenser outlet. Water is left behind in the distillation flask. Acetone boils at 56.5°C while water boils at 100°C. The two components of the mixture separate because acetone is more volatile than water.
Conclusion: The distillation process is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
(Textbook Page 23)
Class 7 Solutions
Activity 2.9
Answer:
Observation: The crystals of pure copper sulpahte in the china dish are observed. The crystals are not alike. The pure crystals can be separated from the liquid in the china dish by filtration.
Conclusion: Pure crystals of copper sulpahte can be obtained from impure sample by the process of crystallisation.
(Textbook Page 25)
Activity 2.10
Answer:
Observation: The material obtained by group 1 is a mixture of the two substances, i.e., iron and sulphur. The material obtained by group 2 is a compound. The properties of the mixture are the same as that of its constituents. We can also observe that the texture and the colour of the compound is the same throughout. A black substance is formed and it does not get attracted to the magnet any more. Hence, group 1 obtained a material with magnetic properties.
Class 7 Solutions
The gas obtained by group 1 is hydrogen gas. It is colourless, odourless and combustible gas. So, it is not advised to do the combustion test for hydrogen in the class. The gas obtained by group 2 is hydrogen sulphide. It is a colourless gas with the smell of rotten eggs. Though the starting materials were same but the product obtained by both the groups showed different properties. The group 1 has carried out the activity involving a physical change whereas in group 2, a chemical change has taken place.
Conclusion: Heat is not needed for the formation of mixture but it is needed for the formation of the compound.
Is Matter Around Us Pure Textbook Questions and Answers
Question 1.
Which separation techniques will you apply for the separation of the following?
(а) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Answer:
(a) Crystallisation or Evaporation
(b) Sublimation
(c) Filtration
(d) Chromatography
(e) Centrifugation
(f) Separating funnel
(g) Filtration
(h) Magnetic separation
(i) Blowing air
(j) Using alum.
Board Exam Guides
Question 2.
Write the steps you would use for making tea. Use the words-solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:
Take the solvent, water, in a kettle, heat it. When the solvent boils, add the solute, milk. Milk and water form a solution. Then pour some tea leaves over a sieve. Slowly pour the hot solution of milk over tea leaves. Colour of tea leaves goes into solution as filtrate. The remaining tea leaves being insoluble remains as residue. Add requisite sugar which dissolves and the tea is ready.
Question 3.
Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (result are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
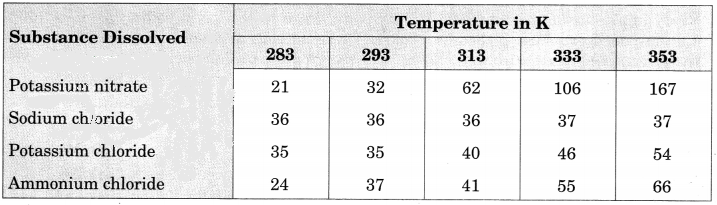
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Answer:
(a) At 313K,
Potassium nitrate needed to produce a saturated solution in 100 grams of water = 62 g
∴ Amount of potassium nitrate to produce a saturated solution in 50 grams of water = 31 g
(b) Some amount of dissolved potassium chloride will reappear as undissolved solid as solubility of solute decreases with the decrease of temperature.
(c) Solubility of each salt at 393 K is as follows:
- Potassium nitrate – 32
- Sodium chloride – 36
- Potassium chloride – 35
- Ammonium chloride – 37
Ammonium chloride salt has the highest solubility at this temperature.
(d) Solubility of a salt increases with the increase in temperature.
Question 4.
Explain the following giving examples.
(a) saturated solution
(b) pure substance
(c) colloid
(d) suspension
Answer:
(а) A solution in which no more solute can be dissolved at a particular temperature is known as a saturated solution. For example, in aqueous solution of sugar no more sugar can be dissolved at room temperature.
(b) A pure substance is a substance consisting of a single type of particles, i.e., all constituent particles of the substance have the same chemical properties. For example, water, sugar, salt, etc.
(c) A colloid is a heterogeneous mixture whose particles are not as small as solution but they are so small that they cannot be seen, with the naked eyes. When a beam of light is passed through a colloid then the path of the light becomes visible. For example, milk, smoke, etc.
(d) A suspension is a heterogeneous mixture in which solids are dispersed in liquids. The solute particles in suspension do not dissolve but remain suspended throughout the medium. For example, paints, muddy water, chalk water mixtures, etc.
Question 5.
Classify each of the following as a homogeneous or heterogeneous mixture: soda water, wood, air, soil, vinegar, filtered tea.
Answer:
(a) Homogeneous mixtures → Soda water, vinegar and filtered tea are homogeneous mixtures. Air is also a homogeneous mixture if dust particles and other suspended impurities are excluded.
(b) Heterogeneous mixtures → Wood and soil are heterogeneous mixtures. Air is also a heterogeneous mixture if dust particles and other suspended impurities are included.
Question 6.
How would you confirm that a colourless liquid given to you is pure water?
Answer:
Every liquid has a characteristic boiling point. Pure water has a boiling point of 100°C (373 K) at 1 atmospheric pressure. If the given colourless liquid boils at even slightly above or below 100°C, then the given liquid is not pure water. Thus, by observing the boiling point, we can confirm whether a given colourless liquid is pure water or not.
Question 7.
Which of the following materials falls in the category of a “pure substance”?
(a) Ice
(b) Milk
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air
Answer:
The following materials fall in the category of a “pure substance”.
(a) Ice
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
Question 8.
Identify the solutions among the following mixtures:
(a) Soil
(b) Seawater
(c) Air
(d) Coal
(e) Soda water.
Answer:
The following mixtures are solutions:
(b) Seawater
(c) Air
(e) Soda water.
Question 9.
Which of the following will show “Tyndall effect”?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution
(d) Starch solution.
Answer:
Tyndall effect is shown by colloidal solutions. Here milk and starch solution are colloids, therefore they will show Tyndall effect.
Question 10.
Classify the following into elements, compounds and mixtures:
(a) Sodium
(b) Soil
(c) Sugar solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(g) Silicon
(h) Coal
(i) Air
(j) Soap
(k) Methane
(l) Carbon dioxide
(m) Blood.
Answer:
Elements: Sodium, silver, tin and silicon.
Compounds: Calcium carbonate, methane and carbon dioxide.
Mixtures: Soil, sugar solution, coal, air, soap and blood.
Question 11.
Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing iron filings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle.
Answer:
The following changes are chemical changes:
(a) Growth of a plant
(b) Rusting of iron
(d) Cooking of food
(e) Digestion food
(g) Burning of a candle.
अध्याय 2: क्या हमारे आस-पास के पदार्थ शुद्ध हैं? – संक्षिप्त नोट्स
1. परिचय
- एक शुद्ध पदार्थ में केवल एक प्रकार के कण (तत्व या यौगिक) होते हैं और इसका एक निश्चित संघटन और गुण होता है।
- एक मिश्रण में एक से अधिक शुद्ध पदार्थ आपस में मिले होते हैं। इसका संघटन परिवर्तनशील होता है।
2. मिश्रण क्या है?
- मिश्रण के प्रकार:
- समांगी मिश्रण: इसका संघटन पूरी तरह से एक समान होता है। इसके घटक दिखाई नहीं देते और आसानी से अलग नहीं किए जा सकते। (जैसे, नमक का घोल, चीनी का घोल, वायु)।
- विषमांगी मिश्रण: इसका संघटन एक समान नहीं होता। इसके घटक दिखाई देते हैं और आसानी से अलग किए जा सकते हैं। (जैसे, नमक और रेत का मिश्रण, कंक्रीट, आइसक्रीम)।
3. शुद्ध पदार्थों के प्रकार
- तत्व: वह पदार्थ जिसे रासायनिक विधियों द्वारा सरल पदार्थों में विभाजित नहीं किया जा सकता। यह केवल एक प्रकार के परमाणु से बना होता है। (जैसे, सोना (Au), ऑक्सीजन (O₂), हाइड्रोजन (H₂))।
- यौगिक: दो या दो से अधिक तत्वों के निश्चित अनुपात में रासायनिक संयोग से बना पदार्थ। इसे केवल रासायनिक विधियों द्वारा ही अपने घटक तत्वों में अलग किया जा सकता है। (जैसे, जल (H₂O), कार्बन डाइऑक्साइड (CO₂), चीनी (C₁₂H₂₂O₁₁))।
- मिश्रण और यौगिक में अंतर:
| गुण | मिश्रण | यौगिक |
|---|---|---|
| संघटन | परिवर्तनशील | निश्चित |
| गुण | घटकों के गुण बने रहते हैं | घटकों से भिन्न नए गुण होते हैं |
| पृथक्करण | घटकों को भौतिक विधियों द्वारा अलग किया जा सकता है | घटकों को केवल रासायनिक विधियों द्वारा अलग किया जा सकता है |
| निर्माण | आमतौर पर कोई ऊर्जा परिवर्तन नहीं होता | ऊर्जा (ऊष्मा, प्रकाश, आदि) का अवशोषण या उत्सर्जन होता है |
4. विलयन, निलंबन और कोलॉइड
मिश्रण को उसके कणों के आकार के आधार पर वर्गीकृत किया जा सकता है।
| गुण | विलयन | निलंबन | कोलॉइड |
|---|---|---|---|
| कणों का आकार | < 1 nm | > 100 nm | 1 nm – 100 nm |
| दृश्यता | दिखाई नहीं देते, प्रकाश को फैलाते नहीं | दिखाई देते हैं, प्रकाश को फैलाते हैं | दिखाई नहीं देते, प्रकाश को फैलाते हैं (टिंडल प्रभाव) |
| समांगिता | समांगी | विषमांगी | विषमांगी परंतु समांगी दिखाई देता है |
| स्थायित्व | स्थायी | अस्थायी (नीचे बैठ जाते हैं) | स्थायी |
| पृथक्करण | छानने द्वारा अलग नहीं किया जा सकता | छानने द्वारा अलग किया जा सकता है | छानने द्वारा अलग नहीं किया जा सकता |
| उदाहरण | पानी में नमक | पानी में रेत | दूध, कोहरा, रक्त |
- मुख्य शब्द:
- विलेय: वह पदार्थ जो घुलता है (जैसे, नमकीन पानी में नमक)।
- विलायक: वह पदार्थ जिसमें विलेय घुलता है (जैसे, नमकीन पानी में पानी)।
- विलयन की सांद्रता: दिए गए विलयन में उपस्थित विलेय की मात्रा।
- द्रव्यमान के प्रतिशत द्वारा:
(विलेय का द्रव्यमान / विलयन का द्रव्यमान) × 100
- द्रव्यमान के प्रतिशत द्वारा:
- संतृप्त विलयन: वह विलयन जिसमें दिए गए तापमान पर और अधिक विलेय नहीं घोला जा सकता।
- टिंडल प्रभाव: कोलॉइडी कणों द्वारा प्रकाश किरण के प्रकीर्णन के कारण प्रकाश के पथ का दृश्यमान होना। यही कारण है कि हम एक धूल भरे कमरे में या कोहरे में सूर्य की किरणों का एक पथ देखते हैं।
5. भौतिक एवं रासायनिक परिवर्तन
- भौतिक परिवर्तन: वह परिवर्तन जिसमें कोई नया पदार्थ नहीं बनता। यह प्रायः उत्क्रमणीय होता है। (जैसे, बर्फ का पिघलना, पानी में चीनी का घुलना)।
- रासायनिक परिवर्तन: वह परिवर्तन जिसमें एक या अधिक नए पदार्थ बनते हैं जिनके नए गुण होते हैं। यह प्रायः अनुत्क्रमणीय होता है। (जैसे, कागज का जलना, लोहे में जंग लगना)।
6. पृथक्करण की विधियाँ
मिश्रण के घटकों को उनके गुणों के आधार पर अलग करने के लिए विभिन्न विधियों का उपयोग किया जाता है।
| विधि | सिद्धांत | उपयोग का उदाहरण |
|---|---|---|
| वाष्पन | अवाष्पशील विलेय को विलायक से अलग करना | समुद्री जल से नमक प्राप्त करना |
| अपकेंद्रण | घूमने से हल्के और भारी कणों को अलग करना | मलाई से मक्खन अलग करना |
| पृथक्कारी कीप | दो अमिश्रणीय द्रवों के घनत्व में अंतर | तेल और पानी को अलग करना |
| उर्ध्वपातन | बिना पिघले ठोस का सीधे वाष्प में बदलना | रेत से अमोनियम क्लोराइड अलग करना |
| क्रोमैटोग्राफी | घटकों की विभिन्न विलेयता और अधिशोषण | स्याही में रंजकों को अलग करना |
| आसवन | क्वथनांक में अंतर | जल का शुद्धिकरण, अल्कोहल और जल को अलग करना |
| प्रभाजी आसवन | मिश्रणीय द्रवों के क्वथनांक में अंतर | पेट्रोलियम के घटकों, वायु को अलग करना |
| क्रिस्टलीकरण | अशुद्ध नमूने से शुद्ध क्रिस्टल प्राप्त करना | नमक या कॉपर सल्फेट का शुद्धिकरण |
- प्रभाजी आसवन: इसका उपयोग तब किया जाता है जब क्वथनांकों का अंतर 25 K से कम होता है। वाष्पीकरण और संघनन के लिए अनेक सतहें प्रदान करने के लिए एक प्रभाजक स्तंभ का उपयोग किया जाता है।
महत्वपूर्ण प्रश्न एवं उत्तर
अति लघु उत्तरीय प्रश्न (1 अंक)
1. विलयन को परिभाषित कीजिए।
उत्तर: विलयन दो या अधिक पदार्थों का एक समांगी मिश्रण होता है। जो घटक कम मात्रा में होता है उसे विलेय और जो अधिक मात्रा में होता है उसे विलायक कहते हैं।
2. टिंडल प्रभाव क्या है?
उत्तर: कोलॉइड में उपस्थित कोलॉइडी कणों द्वारा प्रकाश की किरण के प्रकीर्णन के कारण प्रकाश के मार्ग का दृश्यमान होना, टिंडल प्रभाव कहलाता है।
3. एक गैसीय विलयन का उदाहरण दीजिए।
उत्तर: वायु (गैसों जैसे नाइट्रोजन, ऑक्सीजन, CO₂ आदि का समांगी मिश्रण)।
4. मलाई से मक्खन अलग करने के लिए प्रयुक्त प्रक्रिया का नाम बताइए।
उत्तर: अपकेंद्रण (Centrifugation)।
5. विलयन की सांद्रता क्या है?
उत्तर: दिए गए विलयन में घुले हुए विलेय की मात्रा को विलयन की सांद्रता कहते हैं।
6. संतृप्त विलयन क्या है?
उत्तर: वह विलयन जिसमें दिए गए तापमान पर और अधिक विलेय नहीं घोला जा सकता, संतृप्त विलयन कहलाता है।
7. वायु मिश्रण है या यौगिक?
उत्तर: वायु एक समांगी मिश्रण है क्योंकि इसका संघटन निश्चित नहीं है और इसके घटकों को भौतिक विधियों द्वारा अलग किया जा सकता है।
लघु उत्तरीय प्रश्न (2-3 अंक)
1. समांगी और विषमांगी मिश्रण में उदाहरण सहित अंतर लिखिए।
उत्तर:
| समांगी मिश्रण | विषमांगी मिश्रण |
|---|---|
| संघटन पूरी तरह एक समान होता है। | संघटन एक समान नहीं होता। |
| घटक नग्न आँखों से दिखाई नहीं देते। | घटक दिखाई देते हैं। |
| घटकों के बीच स्पष्ट सीमा नहीं होती। | स्पष्ट सीमा होती है। |
| उदाहरण: नमक का घोल, वायु। | उदाहरण: नमक और रेत का मिश्रण, कंक्रीट। |
2. यौगिक और मिश्रण में अंतर की सूची बनाइए।
उत्तर: (संक्षिप्त नोट्स अनुभाग में दी गई तालिका देखें)। संघटन, गुण, पृथक्करण और ऊर्जा परिवर्तन का उल्लेख करें।
3. आप साधारण नमक और अमोनियम क्लोराइड के मिश्रण को कैसे अलग करेंगे?
उत्तर: हम इन्हें उर्ध्वपातन की प्रक्रिया का उपयोग करके अलग कर सकते हैं। अमोनियम क्लोराइड एक उर्ध्वपाती पदार्थ है, जबकि साधारण नमक नहीं है। मिश्रण को गर्म करने पर, अमोनियम क्लोराइड सीधे वाष्प में बदल जाएगा, जिसे ठंडा करके अलग से एकत्र किया जा सकता है। साधारण नमक पीछे रह जाएगा।
4. जल को सार्वत्रिक विलायक क्यों कहा जाता है?
उत्तर: जल को सार्वत्रिक विलायक इसलिए कहा जाता है क्योंकि यह बहुत अधिक संख्या में पदार्थों (लवण, शर्करा, गैसों, आदि) को घोलकर विलयन बना सकता है। यह इसकी उच्च ध्रुवीयता के कारण होता है।
5. उस विलयन की सांद्रता की गणना कीजिए जिसमें 50g जल में 2g नमक घुला हुआ है।
उत्तर:
- विलेय (नमक) का द्रव्यमान = 2g
- विलायक (जल) का द्रव्यमान = 50g
- विलयन का द्रव्यमान = विलेय का द्रव्यमान + विलायक का द्रव्यमान = 2g + 50g = 52g
- सांद्रता (द्रव्यमान %) = (विलेय का द्रव्यमान / विलयन का द्रव्यमान) × 100
= (2 / 52) × 100 ≈ 3.84%
दीर्घ उत्तरीय प्रश्न (5 अंक)
1. a) क्रोमैटोग्राफी क्या है? इसके सिद्धांत को समझाइए।
b) क्रोमैटोग्राफी का उपयोग करके काले स्याही के घटकों को आप कैसे अलग करेंगे?
उत्तर:
a) क्रोमैटोग्राफी एक ऐसी तकनीक है जिसका उपयोग उन विलेयों को अलग करने के लिए किया जाता है जो एक ही विलायक में घुलनशील होते हैं। इसका सिद्धांत एक मिश्रण के विभिन्न घटकों के एक अधिशोषक (जैसे फिल्टर पेपर) पर अवशोषण में अंतर पर आधारित है। घटक कागज पर अलग-अलग गति से चलते हैं, जिससे वे अलग हो जाते हैं।
b) स्याही को अलग करने की विधि:
- फिल्टर पेपर की एक पट्टी लें और उसके निचले हिस्से के पास काले स्याही से एक रेखा खींचें।
- एक बीकर में थोड़ा पानी (विलायक) डालें, यह सुनिश्चित करें कि पानी का स्तर स्याही की रेखा से नीचे हो।
- पेपर की पट्टी को बीकर में इस तरह लटकाएं कि स्याही का निशान पानी के स्तर से ठीक ऊपर हो।
- जैसे ही पानी पेपर पर ऊपर चढ़ता है, यह स्याही के घटकों को अपने साथ ले जाता है।
- स्याही में मौजूद विभिन्न रंजक पानी में अलग-अलग तरह से घुलेंगे और पेपर पर अलग-अलग तरह से अवशोषित होंगे, जिससे विभिन्न रंगीन बैंड बन जाएंगे।
2. एक नामांकित चित्र के साथ, प्रभाजी आसवन की विधि का वर्णन कीजिए। इसके दो अनुप्रयोग लिखिए।
उत्तर:
- विधि: प्रभाजी आसवन का उपयोग दो या दो से अधिक मिश्रणीय द्रवों के मिश्रण को अलग करने के लिए किया जाता है जिनके क्वथनांकों का अंतर 25 K से कम होता है।
- प्रक्रिया: मिश्रण को एक आसवन फ्लास्क में गर्म किया जाता है। कम क्वथनांक वाले (अधिक वाष्पशील) द्रव की वाष्प पहले उठती है। प्रभाजक स्तंभ में, वाष्पें बार-बार संघनित और पुनः वाष्पित होती हैं। प्रत्येक वाष्पीकरण के साथ, वाष्प अधिक वाष्पशील घटक में समृद्ध होती जाती है। अंत में, अधिक वाष्पशील द्रव की शुद्ध वाष्प संघनित्र में जाती है, जहाँ उसे द्रवित कर एकत्र कर लिया जाता है। कम वाष्पशील घटक फ्लास्क में रह जाता है।
- अनुप्रयोग:
- कच्चे पेट्रोलियम से विभिन्न अंश (जैसे पेट्रोल, डीजल, मिट्टी का तेल) को अलग करना।
- द्रव वायु से विभिन्न गैसों (जैसे ऑक्सीजन, नाइट्रोजन, आर्गन) को अलग करना।
3. भौतिक और रासायनिक परिवर्तनों में अंतर कीजिए। प्रत्येक के तीन उदाहरण दीजिए।
उत्तर:
- भौतिक परिवर्तन: वह परिवर्तन जिसमें कोई नया पदार्थ नहीं बनता और परिवर्तन प्रायः उत्क्रमणीय होता है।
- उदाहरण: बर्फ का पिघलना, कागज का फटना, पानी में चीनी का घुलना।
- रासायनिक परिवर्तन: वह परिवर्तन जिसमें एक या अधिक नए पदार्थ बनते हैं जिनके पूरी तरह से नए गुण होते हैं। परिवर्तन प्रायः अनुत्क्रमणीय होता है।
- उदाहरण: लकड़ी का जलना, लोहे में जंग लगना, भोजन का पकना।